Instrument Integration with LIMS
Posted on : April 8, 2019
By Robert Benz, Sales & Marketing Director for Khemia Software
Laboratories continually find a way to readily retrieve, share, report, and store analytical data from instruments. In prior years, instrument data was printed or handwritten on data sheets, double-checked/verified and passed along to data entry clerks for submission into a reporting system. With improvements in LIMS and instrument software, what was once a drawn out procedure has become a far more automated and less daunting process.
Below are several of the many benefits to the complete integration of instrument data upload to LIMS:
- Reduction in transcription errors
- Creation of an unbroken audit trail
- Greater (much greater!) laboratory efficiency
Any one of these benefits is more than enough to merit instrument integration. The integration process is a worthwhile endeavor for laboratory employees at multiple levels, from analysts and laboratory managers to quality control supervisors to upper management/ownership. It only takes a short time for the entire staff to understand the merits at some direct level.
Upon looking into the decision to automate instrument imports, the first obvious question is: how much will it cost? Unfortunately, that question does not always have a simple answer. The cost can depend on what LIMS you own or are looking to buy; some LIMS vendors charge per instrument setup while others charge per integration type, so as if you have a bench of the same instrument there is only one payment. Additionally, some LIMS companies (Khemia included) have an open-ended integration feature which allows clients to integrate their own instruments, while other LIMS companies keep this locked, denying client access. Another issue to consider is whether or not an instrument essentially counts as one of the named or concurrent users. Although most LIMS vendors do not count an instrument as a user, this does vary by vendor.
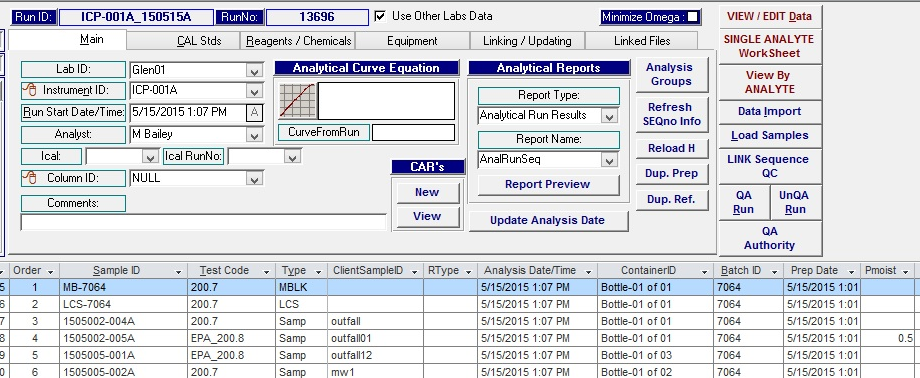
Analytical run in Khemia’s Omega 11 showing the instrument used. Note the Data Import to the right hand side for bringing runs into the LIMS. From this tab, raw data from the instrument may be selected from folders and brought into the LIMS.
Analytical run in Khemia’s Omega 11 showing the instrument used. Note the Data Import to the right hand side for bringing runs into the LIMS. From this tab, raw data from the instrument may be selected from folders and brought into the LIMS.
Once instrument integration has been decided upon, the next concerns which must be addressed are:
-With regard to network security, are instruments allowed on the network?
-Is data only coming from the instrument to the LIMS (unidirectional)?
-Is data intended to be bidirectional? Will both the LIMS and the instrument software allow this?
-Is any middleware (third party software) needed?
-What data is to be imported from the instrument file? Raw or calculated data? QC data? How much
QC data? Blanks, CVS, LCS, Duplicates, etc.?
All of these questions should be thoughtfully considered and often must be answered by the individual laboratory, as the LIMS and/or instruments are capable of answering these questions in different ways. For example, laboratories often have options as to whether or not the instrument software will apply the quality control checks, dry weight corrections and dilution values, or whether that laboratory wants LIMS to handle these calculations internally. Next, where is this data review going to take place? Many laboratories import the raw file and have the LIMS do the data checks and apply the calculations, while others handle this directly on the instrument software. After this host of practical and logistical questions are answered, the final step is the actual integration of the instruments. Fortunately, even rudimentary and older instruments can be integrated with a LIMS provided they have a RS232 or USB port. It is not uncommon to use third party software/middleware in these cases, but this is relatively straightforward.
Unfortunately, there is currently no data standard for laboratory instruments. An emerging data standard covering a range of instrumentation is AnIML (Analytical Information Markup Language). Currently, for many instruments, XML, CSV and PDF are common output types as well as TXT files. It is important for the data be presented in a format that the LIMS can handle. ASCII files, ODBC and proprietary transactions are among the most common methods of transferring data. However, transferring data via ODBC or API requires a full understanding of the LIMS table structure.
There are a number of references that can be utilized to facilitate instrument integration. First, whether using a LIMS currently or looking to purchase a LIMS, LIMS vendors are generally happy to talk with clients and make suggestions based on past experience. At Khemia, we ultimately address this topic in at least eight out of ten live demonstrations. Second, there are some well written, thorough references that help make great guidelines such as ASTM E1578-13 (or -18) Standard Guide for Laboratory Informatics.
In summary, instrument integration with a modern LIMS is an extremely beneficial exercise for which the laboratory will reap substantial rewards in terms of data quality, traceability, and cost effectiveness. Almost every instrument, provided it has at least an RS232 port, may be interfaced with almost every new LIMS. Keep in mind however, that the integration might be unidirectional and not bidirectional, might require third party software and/or might take some time to develop a particular interface.
For any additional information, please contact Robert Benz (rbenz@khemia.com) at Khemia Software, Inc. (www.khemia.com).
