Omega LIMS
Omega LIMS Features
Â
Omega LIMS is a client-server Microsoft Windows application written utilizing a MS Access frontend and a MS SQL Server backend. It is easily configured to use within existing business practices and requires no alterations to laboratory workflow. Omega 11 conforms to multiple standards including ISO 17025, ASTM, NELAC, EPA and DoD-ELAP.
Our LIMS is designed to be an end-to-end solution that handles virtually every phase of laboratory operations and management, allowing users to:
- Schedule sampling and sample pickup
- Track samples throughout their lifecycle (sample checklist, internal chain of custody)
- Automate laboratory operations with a scheduler & workflow designer
- Interface with equipment & instrumentation
- Manage analytical data in a searchable database
- Streamline analytical quality control with built-in data checkers
- Generate final reports, EDDs & data packages
- Track reagents, standards & instrument maintenance throughout lifecycle
- Manage documents, SOPs, accreditations & personnel training records
- Create a paperless laboratory (i.e. EDDs and Reporting)
- Design ad hoc reports to harvest data from SQL tables
- Monitor key performance indicators with management dashboards
Â
Khemia’s Omega LIMS handles every aspect of laboratory operation from prescheduled samples and field data to login, sample preparation and analysis to quality control, reporting and invoicing. Omega LIMS is truly an end to end software solution.
Â
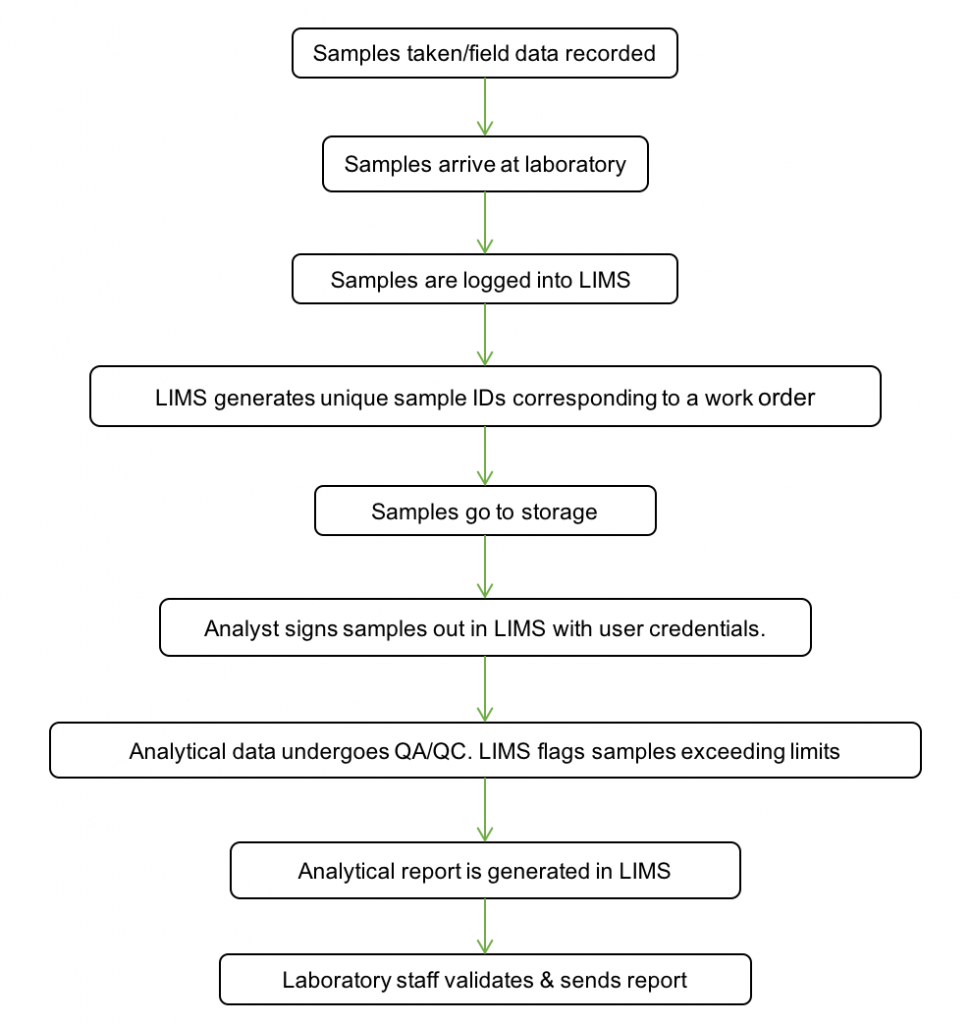
Sample LifecycleÂ
- Field sampling: record field sampling information and notes on the sampling procedure.
- Sample login: samples are logged into the system and given unique ID numbers. They are assigned test methods, quality control criteria, and detection and quantitation levels before moving forward in the lab.
- Sample prep & analysis: record prep information to establish traceability. During analysis, import data from instrumentation or enter manually. Data tables store information in a searchable SQL database.
- Analytical quality control: QC samples exceeding the pre-defined limits are automatically flagged in Omega LIMS. Additional levels of quality review prevent samples from moving forward to the reporting stage without prior authorization by users with QA/QC permissions.
- Reporting: generate analytical reports with a single click. Information presented in reports can be configured based on the client, project, work order or sample group.
- Sample audit trail: view sample transfer history with an internal chain of custody for audit compliance.
Â
General Business ManagementÂ
- Management dashboards: in-depth dashboards allow users to monitor the quantity of samples received by the laboratory, the corresponding dollar value, average turnaround times, on-time percentages, laboratory backlog and more for any given timeframe. The information can be broken down by department, individual staff members, test method, client and more.
- Ad hoc queries: design queries to harvest information from the SQL database.
- User roles configuration: configure user permissions to prevent unauthorized actions.
- Document control: manage SOPs, certifications, staff training records and administrative documents.
- Interface with accounting: Omega LIMS can interface with the most popular accounting systems, including Peachtree, QuickBooks and Sage MAS90.
Â
Laboratory OperationsÂ
- Workflow management: samples logged into the LIMS are automatically added to backlogs of the appropriate laboratory departments based on the test methods required. Backlogs are sorted by holding time, due date or another user-defined parameter. After sample analysis, system protocols require QA/QC review before allowing generation of a final report.
- Scheduler: schedule upcoming tasks and events. Each task can be assigned to individual laboratory personnel and set to repeat at specified intervals. Event reminders can be automatically sent via phone, email, fax, etc. Tasks can be viewed in daily, weekly and monthly calendars and printed for convenience.
- Reagents & standards management: track chemical inventory with unique IDs, attach electronic certificates and note preparation and expiration dates.
- Instrument & equipment management: log manufacturer information, daily usage, calibration, as well as routine and non-routine maintenance.
- Client & vendor information management: store client information, contact details, QA/QC criteria, preferred report formats and more. Vendor information such as commonly purchased items, catalog numbers and unit prices can also be added.
- Interface with instrumentation: import data directly from equipment and instrumentation.
Â
Support
- LIMS training: Omega LIMS clients receive thorough training, both remotely and on-site, by our technology specialists. Remote training sessions are broken down into 16 targeted sessions with the LIMS administrator. On-site training is provided to laboratory staff with appropriate time for Q & A.
- Configuration assistance: LIMS configuration is a guided, step-by-step process with our technical experts and the LIMS administrator.
- Troubleshooting: our Service Agreement includes up to 100 hours of technical support per year.
- Custom programming: offered at client request.
- Software upgrades: regular LIMS upgrades and enhancements are included in the Service Agreement.
Â
